Welcome to our “Molecule of the Month” series, where we dive into the fascinating world of atmospheric pollutants and greenhouse gases that impact our environment and health. This month, we shine the spotlight on Nitrogen Dioxide (NO2), a compound of significant importance due to its adverse effects on:
- The human health: Exposures over short periods can aggravate respiratory diseases and longer exposures to elevated concentrations of NO2 may contribute to the development of asthma and potentially increase susceptibility to respiratory infections.
- The environment: NO2 can interact with water in the atmosphere leading to acid rain.

NO2 is a reactive radical with a boiling point around room temperature (21.2°C) and forms an equilibrium with its dimer N2O4. It is a reddish-brown gas that belongs to the family of nitrogen oxides (NOX). It is primarily formed during high-temperature combustion processes in both natural and anthropogenic sources. Some common sources include:
(I) vehicle emissions, where NO2 is a byproduct of combustion engines in cars, trucks, and other vehicles. Heavy traffic areas are particularly prone to elevated NO2 levels (up to 64% of the total emission);
(II) residential heating systems usually burn fossil fuels releasing NO2. This is especially important for colder regions (around 7% of the total emission);
(III) industrial activities that rely on combustion, such as power plants and factories, contribute to the release of NO2 into the atmosphere (only 3% of the total emission);
(IV) agricultural practices/burnings and fertilizers also play a role in NO2 emissions (rather a local problem).
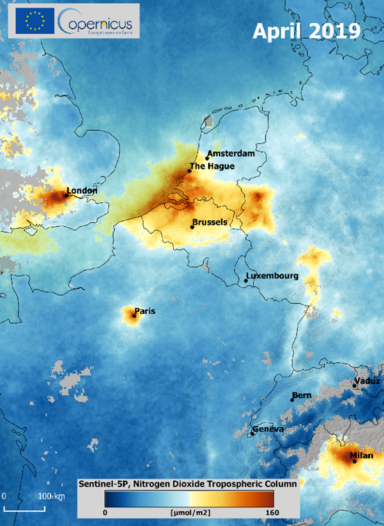
If NO2 emissions are too high and exceeding WHO Global air quality guidelines then drastic measures sometimes need to be taken to protect human’s health, the environment and the climate as it happened in the Netherlands (see fig. 3). The “stikstofcrisis” or nitrogen crisis ripped across Dutch industry after in 2019 the Council of State ruled the government’s strategy for reducing excess nitrogen was in breach on protecting vulnerable habitats. This led to the immediate suspension of various projects as housing, which aggravated the ongoing Dutch housing shortage, plans of drastic reduction of livestock and a reduction of the highway speed limit.
This case in figure 3 shows the importance to monitor nitrogen in the atmosphere (e.g. NO2 and NH3) enabling effective measures to reduce the emissions.
Measurement methodologies
Currently several techniques are used to monitor and quantify NO2, each with its own advantages and disadvantages:
The technique through which NO2 is most widely measured and authorities often trust in is Chemiluminescence (CLD). CLD is an indirect detection method, converting NO2 into NO which, by reacting with ozone, forms an excited state of NO2. The deactivation of this excited NO2 leads to light emission (chemiluminescence). This approach is highly sensitive and can provide real-time measurements for moderate costs. Usually CLD instruments meet the conditions outlined in the EN 14211 standard (“Ambient air – Standard method for the measurement of the concentration of nitrogen dioxide and nitrogen monoxide by chemiluminescence”). CLD’s main disadvantages are the requirement of complex calibration and maintenance due to potential interferences from other nitrogen-containing molecules such as PAN or NH3 and that the detected luminescence signal accounts the sum of ambient NO2 plus NO.
Another frequently used technique is the Differential Optical Absorption Spectroscopy (DOAS). DOAS can provide high sensitivity to NO2 and is valuable for remote sensing applications. It offers insights into NO2 distribution and sources in the atmosphere. But DOAS requires specialized equipment and expertise. Weather conditions and other interfering gases might impact measurements, limiting its utility in certain situations.
Passive or Diffuse Sampling Tubes can be operated without any power source and by non-scientific personnel without technical support. It is a simple, lightweight and cheap technique useful for capturing long-term trends in NO2 concentrations. As it provides averaged measurements, making it less suitable for capturing short-term spikes or variations. Its accuracy might be compromised, particularly in areas with fluctuating NO2 levels.
Electrochemical Sensors offer portability and real-time monitoring capabilities, making them useful for personal exposure assessment to NO2. Cross-sensitivity to other gases and environmental conditions can affect the sensor accuracy. Therefore, sensor calibration and maintenance are crucial for reliable results.
A highly sensitive, selective and precise spectroscopic technique that directly measures gas absorption by analyzing phase shifts in light passing through a high-finesse optical cavity is Cavity Attenuated Phase Shift Spectroscopy (CAPS). To operate the complex and specialized instruments of high initial and operational costs requires complex calibration and validation by skilled users.
Here at MIRO Analytical we are using direct laser absorption spectroscopy in the mid-infrared to monitor and report on air pollutants and greenhouse gases. This technique combines high sensitivity and selectivity by measuring rovibrational transitions allowing accurate identification and quantification of NO2 concentrations even in complex atmospheric mixtures. This makes it arguably the most accurate and versatile method for NO2 monitoring. Advanced instrumentation and data processing techniques make it possible to conduct real-time monitoring with one or ten Hz measurement rates.
Reference projects
The instrument MGA – NO2 was installed at the Jungfraujoch monitoring station of the Swiss National Air Pollution Monitoring Network NABEL (3571 m.a.s.l.) in June 2020 and is reporting live NO2-data since then, convincing Dr. Christoph Hüglin, NABEL/Empa:
“The MIRO laser spectrometer for NO2 operated at the high-alpine site Jungfraujoch performs very well. High sensitivity, precision and reliability combined with the low maintenance make this instrument perfect for our activities at this remote location.”
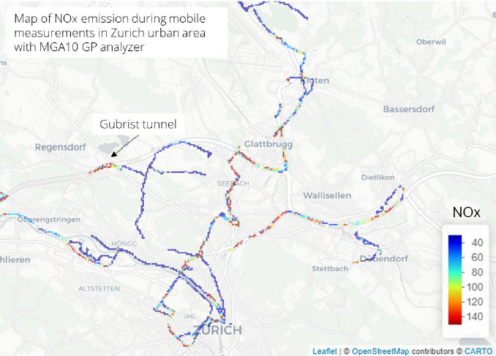
The robustness, mobility, sensitivity, precision and high time resolution of MGA helped to perform in collaboration with the Canton of Zurich (AWEL) a mini-van based mobile real-time monitoring campaign of GHGs and pollutants in cities (for more information, visit Mobile monitoring – MIRO Analytical.
During the measurement campaign, the car travelled around Zurich and continuously measured GHG and pollutants including NO2. The main emitters could be identified.
Conclusion
Nitrogen Dioxide is an essential molecule to monitor due to its adverse effects on human health and the environment. By understanding its sources and utilizing advanced detection techniques, we can work towards a sustainable future with cleaner air and healthier living conditions for generations to come.

As our multi-compound gas analyzers MGA can measure up to ten molecules simultaneously with one single instrument. Join us next month as we explore another molecule shaping our world. And always keep in mind: You can not manage what you cannot measure!