Ammonia (NH₃) is a colorless gas with a pungent smell, widely recognized for its use in agriculture and industry. However, its impact on the environment and air quality often goes unnoticed. This blog entry will explore the sources, effects, and importance of ammonia, as well as the techniques used to measure it.
Ammonia plays a key role in several natural and human-made processes:
Agricultural Use: Ammonia is essential for plant growth and is a major component of fertilizers used in modern agriculture. It provides a vital source of nitrogen, a critical nutrient for crops. However, agricultural activities are also the largest source of ammonia emissions, which occur from the volatilization of nitrogen-based fertilizers and the decomposition of animal waste in livestock farming.
Industrial Applications: Ammonia is a key chemical used in the production of various goods, including fertilizers, cleaning products, and refrigerants. In industrial processes, it also serves as a precursor to explosives and plastics. Despite its widespread use, emissions from industrial activities contribute to the release of ammonia into the atmosphere.
Environmental and Health Concerns: When ammonia is emitted into the atmosphere, it reacts with other pollutants such as nitrogen oxides (NOₓ) and sulfur dioxide (SO₂) to form particulate matter (PM2.5) affecting air quality and visibility. These aerosols can travel long distances, impacting regions far from the original source of emissions. These fine particles can be inhaled, leading to serious health issues. Short-term exposure may cause respiratory irritation, while long-term exposure is linked to chronic respiratory conditions and heart disease. In high concentrations, ammonia is toxic and can be fatal. Ammonia also contributes to soil acidification and water eutrophication, harming ecosystems by reducing biodiversity and damaging aquatic habitats.
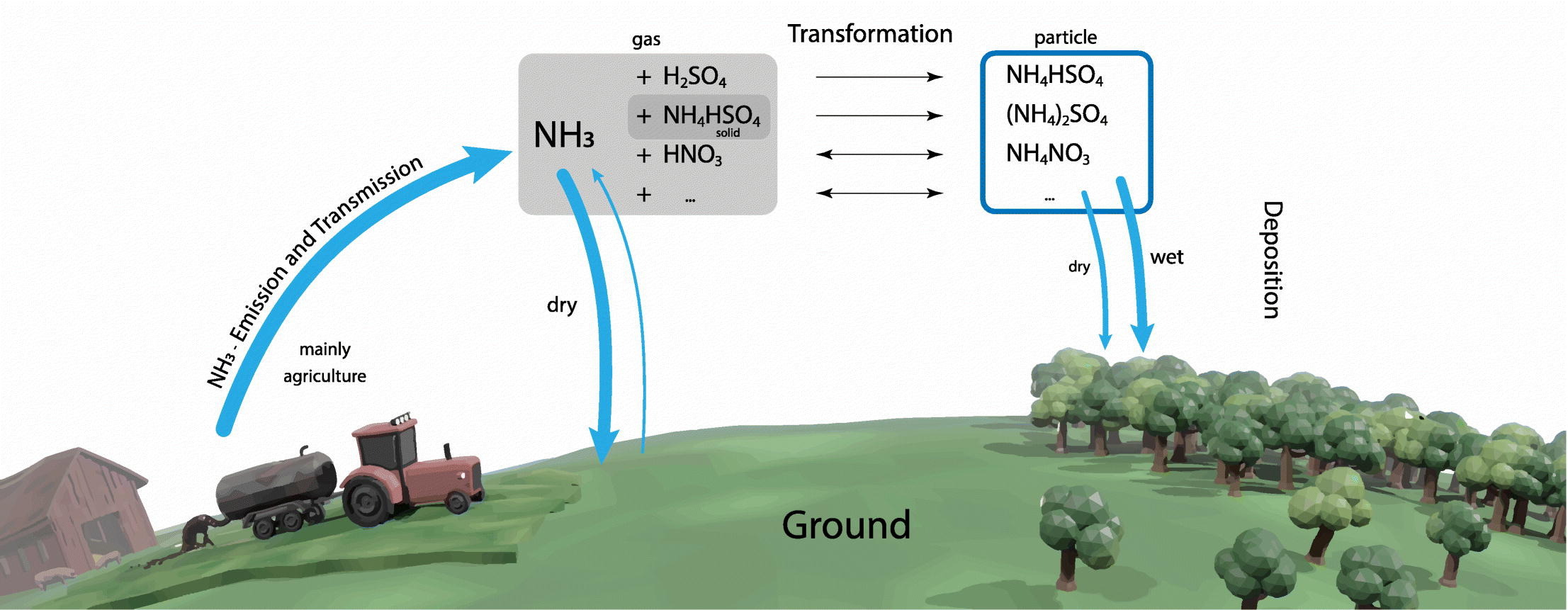
There are two main Sources of atmospheric ammonia:
Agriculture and Livestock: Livestock manure, particularly from large-scale farming operations, is one of the leading sources of ammonia emissions. Ammonia is released when manure breaks down, and its concentration increases in areas with high livestock densities. Additionally, the application of nitrogen-rich fertilizers leads to ammonia emissions from soils, particularly in areas with high temperatures and alkaline pH levels.
Industrial Processes: The production of chemicals, including synthetic ammonia for fertilizers and explosives, is another significant source. Additionally, the combustion of fossil fuels in power plants and vehicles releases ammonia, contributing to its presence in the atmosphere.
To manage ammonia emissions and protect public health, accurate measurement is crucial. Various techniques are used to monitor ammonia in the air:
Electrochemical Sensors: Electrochemical sensors are compact, cost-effective devices that detect ammonia based on the electrochemical reaction of the gas with a specific electrode. These sensors are commonly used in industrial applications and portable monitoring devices. They are advantageous due to their compact size and cost-effectiveness but require regular calibration and have limited sensitivity compared to other techniques.
Photoacoustic Spectroscopy (PAS): PAS measures ammonia by detecting the sound waves produced when ammonia molecules absorb light. This technique offers high sensitivity and is suitable for monitoring ammonia in the atmosphere over a wide range of concentrations. Despite its high sensitivity, the setup is complex and requires skilled operation.
Cavity Ring-Down Spectroscopy (CRDS): CRDS is a highly sensitive technique that measures the absorption of light by ammonia molecules in an optical cavity. This method is particularly useful for detecting low concentrations of ammonia in real-time and is commonly used in environmental monitoring. While it offers high sensitivity and real-time measurements, it requires expensive equipment and skilled operation.
Differential Optical Absorption Spectroscopy (DOAS): DOAS measures ammonia by analyzing the absorption of light at specific wavelengths as it passes through the atmosphere. This technique is capable of measuring multiple gases simultaneously and provides high sensitivity and specificity. However, it involves expensive and complex instrumentation and requires a clear line of sight for accurate measurements.
Chemiluminescence: This technique detects ammonia by measuring the light emitted from a chemical reaction involving ammonia. It is highly sensitive and suitable for continuous monitoring. The equipment is expensive and requires regular calibration and maintenance.
Optical Remote Sensing (ORS): ORS techniques, such as LIDAR, use laser light to detect ammonia over large areas. These methods are non-intrusive and can cover extensive regions. They are advantageous for covering large areas but require expensive and complex instrumentation and clear atmospheric conditions.

Here, at MIRO Analytical, we use direct laser absorption spectroscopy (LAS) in the mid-infrared region to monitor NH3 together with up to 9 other gases. This technique offers highest sensitivity, selectivity, and real-time monitoring capabilities, making it ideal for trace-level, real-time continuous monitoring. As a robust multi-compound gas analyzer with high time resolution (up to10 Hz), MIRO´s MGA is ideal for measurements in the atmosphere. LAS’ precision, wide dynamic range, non-destructive nature and the direct quantification without conversion needed, contribute to its effectiveness in providing accurate and reliable data.
Ammonia may not always receive the attention it deserves as an air pollutant, but its impact on both human health and the environment is profound. From agriculture to industry, ammonia plays an essential role in modern life, yet its emissions contribute to air pollution, ecosystem damage, and health risks. By understanding ammonia’s sources, effects, and the methods used to measure it, we can take steps to mitigate its harmful impacts and ensure cleaner air for future generations.
Stay tuned for more explorations into the molecules shaping our world and remember — you cannot manage what you cannot measure!