Welcome to the last edition of our “Molecule of the Month” series! This time, we focus on nitrous oxide (N₂O), a critical atmospheric trace gas with significant implications for both climate change and stratospheric ozone
chemistry. Often overlooked in discussions about greenhouse gases, N₂O is one of the most potent contributors to global warming and plays a key role in the depletion of the ozone layer.

Nitrous oxide is a molecule of significant importance due to its impact on climate change, ozone depletion, and its agricultural and industrial relevance.
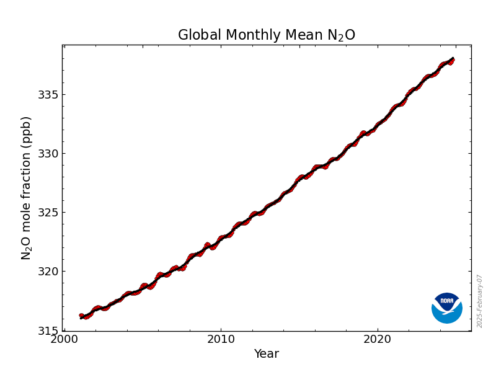
A Potent Greenhouse Gas: Although less abundant than carbon dioxide (CO₂) and methane (CH₄), nitrous oxide is nearly 300 times more effective at trapping heat in the atmosphere over a 100-year period. It has a long atmospheric lifetime of around 120 years, making it a significant driver of climate change.
Ozone Layer Depletion: Unlike CO₂ and CH₄, N₂O is not only a greenhouse gas but also a leading ozone-depleting substance. Once it reaches the stratosphere, N₂O reacts with oxygen atoms to form nitric oxide (NO), which participates in catalytic reactions that break down ozone (O₃).
Agricultural and Industrial Relevance: N₂O emissions are closely linked to human activities, particularly in agriculture. Understanding and mitigating its emissions are crucial steps in reducing both climate and environmental impacts.
The importance of nitrous oxide underscores the need for accurate measurement and monitoring. This brings us to the methods used for N₂O flux measurements, which are essential for understanding its emissions and developing effective mitigation strategies.
N₂O flux measurements involve quantifying the N2O levels emitted from various sources, primarily soils. The most common methods include soil incubation chambers, which capture emissions in small, enclosed chambers, and micrometeorological methods like eddy covariance, which measure gas fluxes over larger areas. These measurements are crucial to study agricultural practices or wet- and peatlands. Accurate data from these measurements covering small variations in the N2O levels of less than 2 ppb, support the development of policies and regulations aimed at reducing greenhouse gas emissions and combating climate change.
N₂O flux measurements depend heavily on the sources of N₂O emissions. Let’s explore these sources:
Natural Sources:
Microbial Processes in Soil and Water: The majority of natural N₂O emissions come from microbial nitrification and denitrification in soils and oceans.
Forest Fires: Biomass burning releases N₂O into the atmosphere.
Oceans: Marine environments contribute to global N₂O emissions through microbial processes in surface and deep waters.
Anthropogenic Sources:
Agriculture (Fertilizer Use): The largest human source of N₂O emissions is the excessive use of nitrogen-based fertilizers, which enhance microbial production of N₂O in the soil.
Industrial Processes: Chemical manufacturing, particularly nitric acid and adipic acid production, contributes to N₂O emissions.
Fossil Fuel Combustion: Transportation, power plants, and other combustion-related activities release N₂O into the atmosphere.
Wastewater Treatment: Organic matter breakdown in wastewater treatment plants also produces N₂O.
A powerful tool for identifying major sources of N₂O emissions is isotopic analysis. By examining isotopic ratios, researchers can distinguish between different sources and processes that produce N₂O. For instance, isotopic signatures can help differentiate between N₂O emissions from agricultural soils, industrial activities, and natural processes.
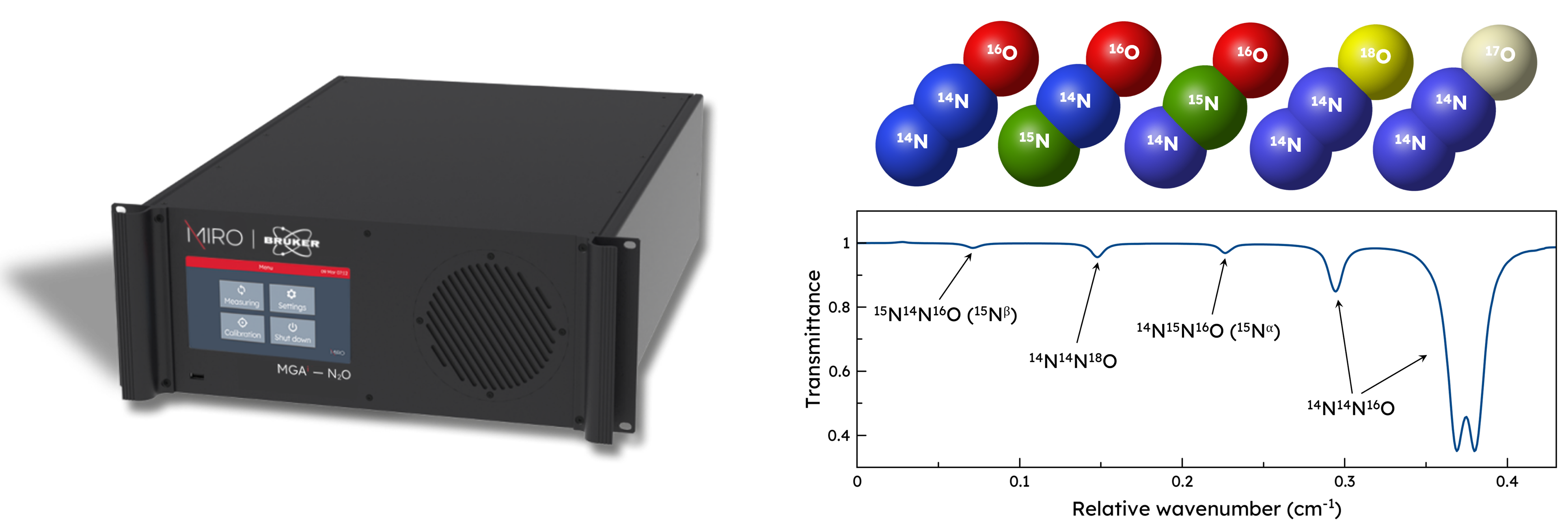
Rovibrational spectroscopy is particularly suitable for this purpose because it can distinguish between 15N14N16O and 14N15N16O by their different rotational behaviors. These two isotopes have the exact same mass and therefore are not distinguishable by mass spectrometry. MIRO’s isotopic analyzer MGAi – N₂O measures the isotopic ratios of up to five isotopes including 15N14N16O and 14N15N16O as. This capability provides detailed insights into the microbial pathways responsible for N₂O production.
Monitoring N₂O in the atmosphere is crucial, however, it is challenging due to its relatively low concentration, small variations over time, and the lack of inexpensive techniques available for accurate detection. Here are some common techniques used:
Gas Chromatography (GC): A traditional and widely used technique that often uses an electron capture detector (ECD) to measure N₂O concentrations. It offers high sensitivity and resolution, allowing for the detection of very low concentrations of N₂O, and is capable of separating complex mixtures of gases. However, it is only suitable for volatile and thermally stable compounds, requires regular calibration and maintenance, and is destructive to the sample, except when using mass spectrometry detectors.
Cavity Ring-Down Spectroscopy (CRDS): A highly sensitive method that measures the time it takes for light to decay in a cavity containing the gas sample. It offers high sensitivity and is suitable for continuous monitoring. However, it has a high initial cost and complexity of setup, requires precise alignment of optical components, and is sensitive to environmental conditions like temperature and pressure.
Off-Axis Integrated Cavity Output Spectroscopy (OA-ICOS): Uses a laser to measure gas concentrations with high precision. It offers high sensitivity and precision, is less sensitive to alignment issues and environmental changes, and is suitable for remote and harsh environments. The drawbacks include high initial cost, lower output intensity compared to other methods, and the need for specialized equipment and expertise.
Here, at MIRO Analytical, we use direct laser absorption spectroscopy (LAS) in the mid-infrared region to measure up to 5 isotopes of N₂O or together with up to 9 other gases. This technique offers highest sensitivity, selectivity, and real-time monitoring capabilities, making it ideal small variations, 10 Hz measurements and real-time continuous monitoring.
Conclusion
Nitrous oxide may not be as widely discussed as CO₂ or CH₄, but its impact on climate change and ozone depletion makes it a molecule of major concern. With its long atmospheric lifetime and powerful greenhouse effect, reducing N₂O emissions—especially from agriculture and industry—is crucial for protecting both our climate and the ozone layer. Isotopic analysis plays a key role in identifying emission sources by examining the isotopic composition of N₂O, allowing researchers to distinguish between different sources and processes.
Thank you for following the “Molecule of the Month” blog series! Your curiosity and engagement have made this journey truly rewarding. Stay curious and keep exploring the fascinating world of chemistry!