Welcome to this month’s edition of “Molecule of the Month,” where we spotlight the diverse and impactful molecules that shape our atmosphere and world. This month, we turn our attention to ethylene oxide (EtO), a versatile and reactive compound with significant industrial, environmental, and health implications. Join us as we explore the sources, uses, and measurement techniques of ethylene oxide.
In contrast to the other gases discussed in this blog, EtO is almost exclusively a man-made atmospheric component. EtO is a colorless, flammable gas with a slightly sweet odor. It is a volatile organic compound (VOC) with the formula C2H4O. EtO is a cyclic ether and the simplest epoxide, consisting of a three-membered ring made up of one oxygen atom and two carbon atoms. This three-membered ring structure contributes to its high reactivity, making it both a valuable industrial chemical and a potential health hazard. Here’s why EtO is important:
Industrial Applications: EtO is a crucial intermediate in the production of numerous chemicals, including ethylene glycol (used in antifreeze and polyester production), surfactants, and ethanolamines. Its ability to sterilize medical equipment, owing to its bactericidal and sporicidal properties, makes it indispensable in healthcare.

Health Impact: Despite its industrial benefits, EtO is classified as a human carcinogen by various health organizations, including the International Agency for Research on Cancer (IARC). Long-term exposure can lead to increased risks of lymphoid and breast cancers. Short-term exposure can cause respiratory irritation, headaches, and nausea.
Environmental Impact: Research shows that EtO is present in outdoor air across the United States with an average concentration of approximately 0.2 ppb. EtO emissions can contribute to air pollution and pose risks to environmental health. Its estimated half-life in the atmosphere ranges from 70 days during summer months to 150 days during winter months. When released into the atmosphere, EtO reacts to form formic acid, a naturally occurring chemical and contributes to the formation of ground-level ozone, affecting air quality and ecosystems.
Sources:
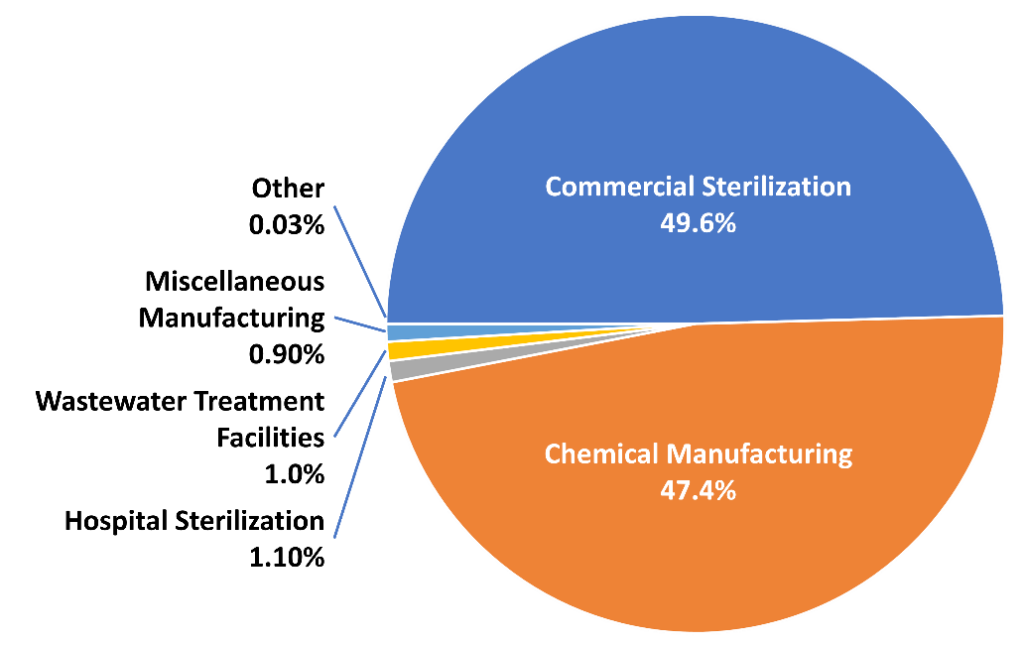
Industrial Emissions: The primary sources of EtO are industrial facilities where it is produced or used, such as chemical manufacturing plants and sterilization facilities. These emissions can impact the air quality in surrounding areas, posing health risks to nearby communities.
Accidental Releases: Accidental releases during transportation or from storage tanks can lead to localized spikes in EtO concentrations, necessitating emergency response measures to protect public health and safety.
New EPA regulations from April 2024
The U.S. Environmental Protection Agency (EPA) has recently tightened regulations on EtO emissions. These rules apply to a variety of industrial sources, including sterilizers and chemical-manufacturing facilities. A significant part of these regulations is the obligation for manufacturers to monitor EtO emissions. The EPA now requires facilities to employ systems for continuous monitoring and regularly report this data. If levels surpass 10 parts per billion (ppb), workers would be required to wear Personal Protective Equipment (PPE). These measures aim to protect public health and the environment from the risks associated with EtO.
Accurate measurement of ethylene oxide concentrations is crucial for regulatory compliance, environmental monitoring, and protecting public health. Various techniques are used to detect and quantify EtO:
1. TO-15/TO-15A Method: This method uses canisters as sampling media and Gas Chromatography/Mass Spectrometry (GC/MS) as the analytical instrument for EtO measurements. It’s a widely adopted and effective method for Volatile Organic Compounds (VOC) analysis of air samples but during the time of sample collecting on site and bring it to the lab for analysis the concentrations might change.
Fourier Transform Infrared (FTIR) Spectroscopy: FTIR spectroscopy detects EtO by measuring the absorption of infrared light at specific wavelengths. This method is non-destructive and provides real-time measurements. It is effective for continuous monitoring in industrial settings but can be affected by the presence of other gases and might lack of sensitivity.
Photoionization Detectors (PIDs): PIDs measure EtO by ionizing the gas with ultraviolet light and detecting the resulting ions. These detectors are portable and provide rapid, real-time measurements, making them useful for field monitoring and emergency response. However, they can be less selective, requiring calibration and potential cross-sensitivity to other volatile organic compounds (VOCs).
Colorimetric Tubes: Colorimetric tubes are a simple and cost-effective method for detecting EtO. They involve drawing air through a tube containing a chemical reagent that changes color in the presence of EtO. While easy to use, they are less sensitive and accurate compared to more sophisticated techniques, making them suitable for preliminary screening rather than precise quantification.
Laser Spectroscopy: Several laser spectroscopy methods are available to monitor EtO at ultra-trace levels. Laser spectroscopy measures the absorption of laser light by the target molecule. Laser systems can provide accurate, real-time measurements of EtO.
Here, at MIRO Analytical, we can use direct laser absorption spectroscopy (LAS) in the mid-infrared region to monitor EtO together with up to 9 other gases. This technique offers highest sensitivity, selectivity, and real-time monitoring capabilities, making it ideal for trace-level, real-time continuous monitoring. As a robust multi-compound gas analyzer with high time resolution (up to10 Hz), MIRO´s MGA is ideal for industrial applications. LAS’ precision, wide dynamic range, and non-destructive nature contribute to its effectiveness in providing accurate and reliable data. Furthermore, it’s direct measurements of EtO are less susceptible to interference from other gases.

Conclusion:
Ethylene oxide is a multifaceted molecule with significant industrial utility and potential health hazards. Understanding its sources, effects, and measurement techniques is essential for balancing its benefits and risks. Effective monitoring and regulation of EtO emissions can help protect public health and the environment, ensuring that this valuable compound is used safely and responsibly. Stay tuned for our next exploration into the intriguing world of molecules that shape our lives, atmosphere and our planet! And always remember, you cannot manage what you cannot measure!