In our latest exploration of atmospheric compounds, we spotlight water vapor (H₂O), an essential component of Earth’s atmosphere that profoundly influences weather, climate, and life itself. While it is often overshadowed by greenhouse gases like carbon dioxide and methane, water vapor plays an indispensable role in regulating our planet’s climate and hydrological cycles.
There are several reasons why in the atmospheric context water vapor is important:
Climate Regulation: Water vapor is Earth’s most abundant greenhouse gas, trapping heat and maintaining the planet’s temperature. This natural “greenhouse effect” allows Earth to sustain life as we know it. However, water vapor also amplifies climate change through a feedback loop: as temperatures rise, more water evaporates into the atmosphere, intensifying the greenhouse effect.
Weather and Precipitation: Water vapor drives weather patterns by forming clouds, rain, and snow. As water vapor condenses in the atmosphere, it releases latent heat, fueling storm systems and influencing wind patterns. Without water vapor, Earth’s weather systems would grind to a halt.
Energy Transfer: Water vapor plays a critical role in transferring energy within Earth’s climate system. Evaporation at the surface absorbs heat, while condensation in the atmosphere releases it. This process helps distribute energy across the planet, moderating temperature extremes.
Impact on Air Quality: Water vapor interacts with other atmospheric compounds, influencing the formation of aerosols, secondary pollutants, and even ozone. Its role in atmospheric chemistry makes it a crucial factor in air quality.
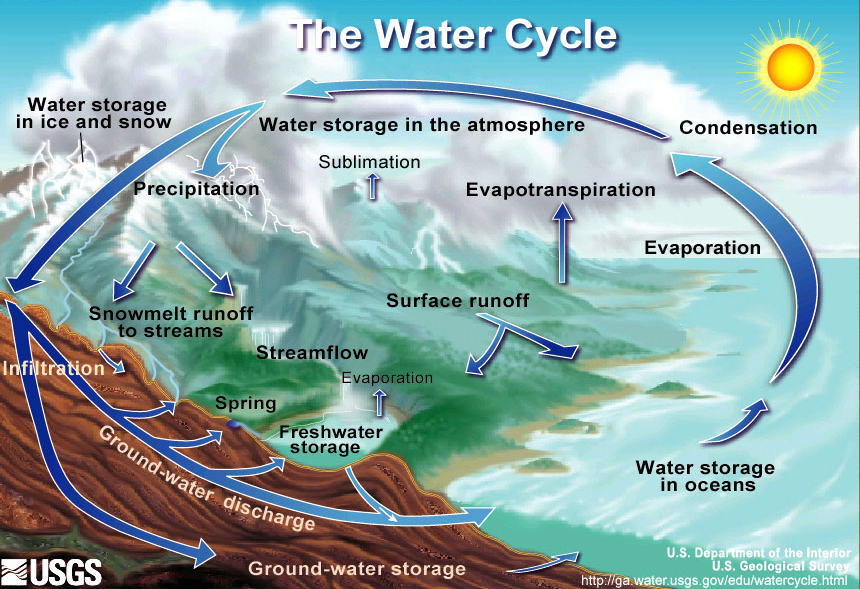
There are different sources of water vapor and its distribution varies:
1. Natural Sources: Most water vapor enters the atmosphere through natural processes like evaporation from oceans, lakes, and rivers, as well as transpiration from plants. Oceans are the primary source, accounting for over 70% of global evaporation.
2. Human Influence: While human activities do not directly emit significant amounts of water vapor compared to natural sources, activities like irrigation and industrial cooling can locally increase atmospheric moisture levels.
3. Geographical Variation: The concentration of water vapor varies widely with temperature and location. Tropical regions, with their warm temperatures and abundant surface water, have the highest levels of atmospheric moisture, while polar regions are much drier.
Accurately measuring water vapor is crucial for weather forecasting, climate modeling, and understanding atmospheric processes. Advanced techniques include:
Hygrometer: This is the most common device for measuring humidity. There are different types of hygrometers, including digital and analog versions. Digital hygrometers use sensors to measure electrical changes caused by moisture, while analog hygrometers often use materials that expand or contract with humidity changes
Psychrometer: This device uses two thermometers, one with a dry bulb and one with a wet bulb. The difference in temperature readings between the two bulbs can be used to calculate the relative humidity
Dew Point Hygrometer: This instrument measures the dew point, which is the temperature at which air becomes saturated with moisture and dew forms. It often uses a chilled mirror to detect condensation
Capacitive Sensors: These sensors measure humidity by detecting changes in capacitance caused by moisture in the air. They are commonly used in modern electronic hygrometers

Here, at MIRO Analytical, we use direct laser absorption spectroscopy (LAS) in the mid-infrared region to measure water vapor together with up to 9 other gases. This technique offers highest sensitivity, selectivity, and real-time monitoring capabilities, making it ideal for trace-level, real-time continuous monitoring.
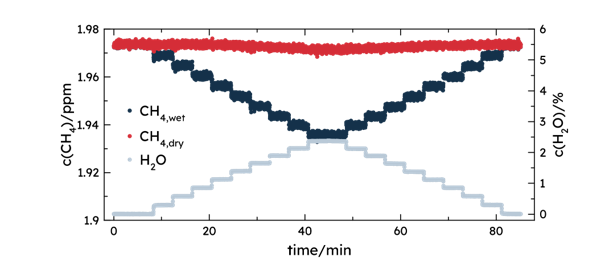
The water vapor measurements are also used for a water-vapor correction, which is crucial for accurate GHG measurements for several reasons. Firstly, many atmospheric models and inventories require GHG measurements to be reported as dry air mole fractions. This standardization is necessary because the variability in water vapor can mask the signals of the greenhouse gases, making it difficult to compare data across different conditions and locations. Additionally, water vapor can dilute the concentration of GHGs in the atmosphere. When humidity levels change, the concentration of these gases appears to change as well. Correcting for water vapor ensures that the measurements reflect the true concentration of the gases, independent of humidity variations. Finally, water vapor can interfere with the spectral lines used to detect GHGs, leading to inaccuracies in the measurements. By applying a water-vapor correction, the spectral data can be adjusted to account for this interference, resulting in precise readings within the experimental uncertainty.
Conclusion:
Water vapor, while often overlooked, is the lifeblood of Earth’s atmospheric system. From shaping weather patterns to amplifying climate change, its role is both complex and indispensable. Understanding and monitoring water vapor is critical for predicting weather, managing water resources, and mitigating the impacts of climate change. Furthermore, a complete water vapor correction is essential for reporting accurate GHG concentrations. Stay tuned for more insights in our “Molecule of the Month” series as we continue to uncover the vital roles of atmospheric compounds shaping our world — you cannot manage what you cannot measure!
About the author: Dr Jonas Bruckhuisen studied chemistry at the RWTH University in Aachen, Germany, before obtaining his PhD in gas phase spectroscopy and atmospheric science from the Université du Littoral Côte d’Opale in Dunkirk, France. He has been application scientist at MIRO Analytical since 2023.