Welcome to this month’s entry in our exploration of atmospheric compounds! Today, we’re focusing on nitrous acid (HONO), a trace gas with a powerful role in atmospheric chemistry. Although present in low concentrations, HONO significantly influences air quality, public health, and the formation of pollutants. Let’s dive into the importance of HONO, its sources, impacts, and the advanced methods used to detect it.

Though HONO exists in trace amounts, it plays a critical role in the atmosphere. Here’s why it matters:
Photochemical Activity and Hydroxyl Radical (OH) Production: HONO is a precursor of hydroxyl radicals (OH), often referred to as the “atmosphere’s detergent” due to their role in breaking down pollutants and greenhouse gases like methane. When sunlight hits HONO, it quickly photolyzes to form OH radicals, initiating and sustaining chemical reactions that transform pollutants and influence O3 production.
Ozone Formation: OH radicals generated from HONO contribute to ground-level O3 formation, a key component of urban smog. While stratospheric O3 protects life on Earth from UV radiation, O3 near the ground level harms respiratory health and agricultural productivity.
Health Impacts: HONO is not only reactive but also potentially harmful itself. Short-term exposure to HONO can irritate the respiratory system, while long-term exposure is linked to respiratory diseases and other health issues. HONO’s ability to initiate photochemical reactions that form secondary pollutants only adds to its public health implications.
The main HONO sources are:

1. Anthropogenic Sources: HONO is emitted by human activities, especially through combustion processes. Vehicles, industrial processes, and biomass burning release nitrogen oxides (NOx), which react on surfaces to produce HONO. Urban areas with high NOx emissions tend to have elevated HONO levels, especially in the morning when sunlight begins the photolysis process.
2. Natural Sources: Although less is known about natural HONO emissions, it is believed to form through soil microbial activity, reactions on soil surfaces, and during photolytic processes on organic materials like plants and soil. Wet surfaces, such as leaves and soil, are active sites where HONO can form through natural processes.
3. Secondary Formation: In addition to direct emissions, HONO forms through secondary reactions in the atmosphere. Nitrogen dioxide (NO₂) reacts with water vapor on surfaces or aerosols to produce HONO. This heterogeneous formation pathway is especially relevant in urban areas, where nitrogen dioxide is abundant.
Urban air quality:
HONO is a particularly urban topic due to several factors that are unique to urban environments. Urban areas have numerous sources of HONO, including vehicle emissions, industrial activities, and biomass burning. These sources are more concentrated in cities, leading to higher levels of HONO compared to rural areas. Urban environments provide a variety of surfaces, such as buildings, roads, and other infrastructure, where nitrogen dioxide (NO₂) can react to form HONO. These heterogeneous reactions are more prevalent in cities due to the abundance of these surfaces. Urban areas can have unique microclimates with higher humidity and temperature variations, which can influence the formation and concentration of HONO. For example, higher humidity can enhance the conversion of NO₂ to HONO on surfaces. The high levels of HONO in urban areas contribute significantly to the formation of secondary pollutants, affecting air quality and public health.
This makes understanding and controlling HONO emissions particularly important for urban air quality management. A transportable HONO analyzer is crucial for capturing the spatial variability and real-time dynamics of HONO in urban areas. It allows for flexible deployment in various locations, providing comprehensive data for effective air quality management.
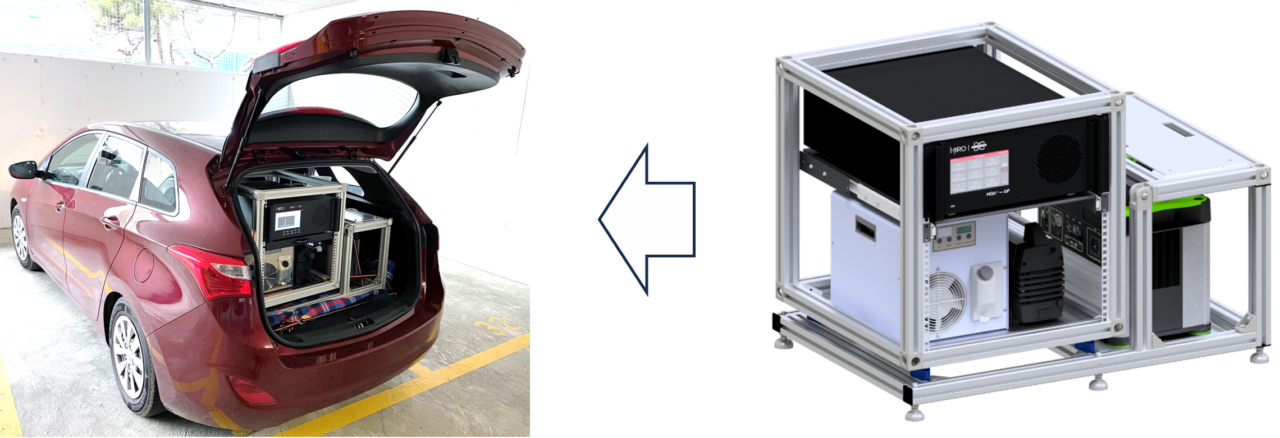
Due to its highly reactive and transient nature, measuring HONO accurately is challenging. However, several advanced methods have been developed to detect HONO at trace levels:
Differential Optical Absorption Spectroscopy (DOAS): This method uses the absorption of light to measure HONO concentrations over long paths in the atmosphere. It’s widely used due to its accuracy and ability to cover large areas. It offers high accuracy and can measure multiple trace gases simultaneously. It is non-intrusive and provides continuous monitoring. However, it requires complex equipment and calibration and is sensitive to atmospheric conditions like humidity and aerosols, which can affect measurements.
Long-Path Absorption Photometer (LOPAP): A wet chemical technique that involves drawing air through a solution that reacts with HONO, allowing for its quantification. LOPAP is known for its high sensitivity and accuracy in detecting low concentrations of HONO. It can provide real-time, in situ measurements and is less prone to interferences from other gases. On the downside, it can have potential chemical interferences and long response times for some applications, and it requires regular maintenance and calibration.
Chemical Ionization Mass Spectrometry (CIMS): This technique ionizes HONO molecules and measures their mass-to-charge ratio. It’s highly sensitive and can provide detailed chemical information. It is capable of detecting very low concentrations of HONO, has a fast response time, and offers high selectivity. However, it requires expensive and complex instrumentation, and calibration can be challenging due to the need for specific chemical standards.

Here, at MIRO Analytical, we use direct laser absorption spectroscopy (LAS) in the mid-infrared region to monitor HONO together with up to 9 other gases. This technique offers highest sensitivity, selectivity, and real-time monitoring capabilities, making it ideal for trace-level, real-time continuous monitoring. As a robust multi-compound gas analyzer with high time resolution (up to10 Hz), MIRO´s MGA is ideal for measurements in the atmosphere. LAS’ precision, wide dynamic range, non-destructive nature and the direct quantification without conversion needed, contribute to its effectiveness in providing accurate and reliable data.
Nitrous acid (HONO) may be a trace compound, but its influence on atmospheric chemistry is anything but minor. By fueling the production of hydroxyl radicals, HONO plays a crucial role in pollutant degradation, O3 formation, and, ultimately, air quality. Given its importance and reactivity, measuring and understanding HONO concentrations in urban but also in rural environments is essential for protecting public health and modeling air pollution accurately.
As we continue to study this fascinating compound, we gain new insights into the complex chemistry that shapes our atmosphere and, ultimately, our health. Stay tuned for more updates in our “Molecule of the Month” series, where we explore the invisible but impactful components of our world! Because — you cannot manage what you cannot measure!
About the author: Dr Jonas Bruckhuisen studied chemistry at the RWTH University in Aachen, Germany, before obtaining his PhD in gas phase spectroscopy and atmospheric science from the Université du Littoral Côte d’Opale in Dunkirk, France. He has been application scientist at MIRO Analytical since 2023.