Ozone (O₃) is a unique molecule with a split personality: it plays both a protective and a harmful role depending on where it is found in the atmosphere. This month, we explore the fascinating dual nature of ozone, its significance for our planet, and the techniques used to measure it.

The Intergovernmental Panel on Climate Change (IPCC), the Environmental Protection Agency (EPA), and the World Meteorological Organization (WMO) all recognize ozone’s role in the atmosphere. Nevertheless, the EPA and WMO do not classify O₃ as an official GHG like carbon dioxide (CO₂) or methane (CH₄) due to its complex and variable role in the atmosphere. Officially classified GHGs are typically those that have a well-defined and consistent impact on the greenhouse effect and are present in significant quantities globally. However, O₃ still contributes to the greenhouse effect. In the lower atmosphere (troposphere), ozone acts as a GHG by trapping heat, which can lead to warming. In the upper atmosphere (stratosphere), ozone plays a crucial role in blocking harmful ultraviolet (UV) radiation from the sun.
Ozone’s importance varies dramatically based on its location in the atmosphere:
Stratospheric/Good Ozone: In the stratosphere, roughly 10 to 30 kilometers above Earth’s surface, ozone forms the famous ozone layer. This layer acts as a shield, absorbing the majority of the Sun’s harmful UV radiation, particularly UV-B and UV-C rays. Without this protective layer, life on Earth would be exposed to dangerous levels of UV radiation, leading to increased rates of skin cancer, cataracts, and other health issues, as well as harmful effects on ecosystems.
Tropospheric/Bad Ozone: At ground level, in the troposphere, ozone is a harmful air pollutant and a key component of smog. Unlike stratospheric ozone, which is formed naturally, tropospheric ozone is created by chemical reactions between volatile organic compounds (VOCs) and nitrogen oxides (NOₓ) in the presence of sunlight. This “bad” ozone is a powerful oxidant that can cause respiratory problems, aggravate asthma, reduce lung function, and damage crops, forests, and other vegetation. Therefore, the US EPA has set the National Ambient Air Quality Standards (NAAQS) for ozone at 70 ppb, averaged over an 8-hour period.
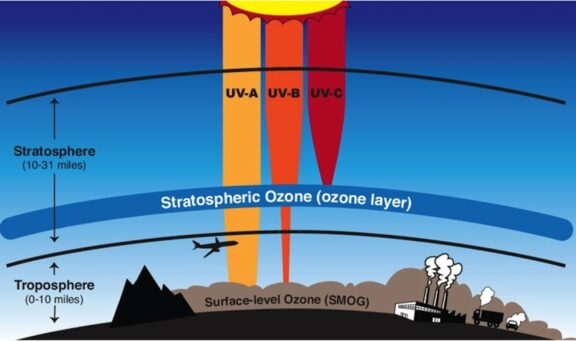
There are three main Sources/Effects worth mentioning:
1. Stratospheric Ozone Depletion: Human activities, particularly the emission of chlorofluorocarbons (CFCs) and other ozone-depleting substances, have led to the thinning of the ozone layer, most notably creating the “ozone hole” over Antarctica. While international efforts, such as the Montreal Protocol, have significantly reduced the use of these substances, the ozone layer’s recovery remains a slow process, with full recovery expected around the middle of this century.
2. Ground-Level Ozone Formation: Ground-level ozone is primarily formed by the reaction of sunlight with pollutants such as nitrogen oxides (NOₓ) and VOCs, emitted by vehicles, industrial facilities, and other sources. This form of ozone contributes to the formation of photochemical smog, particularly during the summer months. It is a significant pollutant in urban areas and can be transported long distances by wind, affecting air quality far from its sources.
3. Health and Environmental Impact: Ground-level ozone is particularly concerning due to its impact on human health. It can cause or exacerbate respiratory diseases, reduce lung function, and lead to hospital admissions. Ozone exposure is also harmful to sensitive vegetation and ecosystems, reducing crop yields and affecting the health of forests.
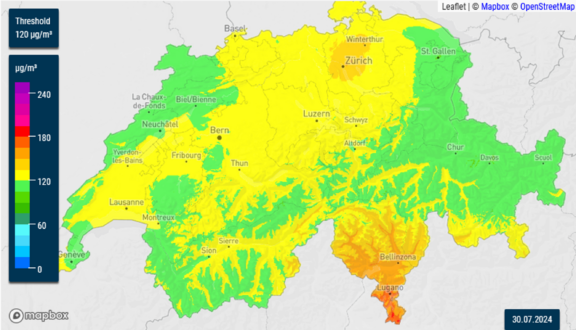
Accurately measuring ozone concentrations is critical for monitoring air quality, studying atmospheric processes, and protecting public health. Various techniques are used for ozone detection:
UV Absorption Spectroscopy: This technique measures the absorption of ultraviolet light by ozone molecules, with the amount of absorbed light corresponding to the ozone concentration. It’s highly accurate and widely used, though it requires calibration and can be affected by other UV-absorbing substances.
Chemiluminescence: In this method, ozone reacts with a reagent to produce light, and the intensity of this light is proportional to the ozone concentration. It’s sensitive and specific to ozone, but it needs consumable reagents and can be influenced by other reactive gases.
Electrochemical Detection: This technique uses a sensor with an electrolyte that reacts with ozone, generating an electrical current proportional to the ozone level. It’s portable and relatively inexpensive, though the sensors have a limited lifespan and can be affected by other gases.
Differential Optical Absorption Spectroscopy (DOAS): DOAS measures the absorption and compares the measured spectrum to a known absorption pattern. That way the concentration of gases like ozone are determined, making it suitable for both ground-based and remote sensing. It can measure multiple gases simultaneously, but it requires complex data analysis and calibration.
Light Detection and Ranging (LIDAR): This method uses laser pulses to measure the distance to and concentration of ozone in the atmosphere by analyzing backscattered light. It provides high-resolution vertical profiles of ozone, although it is expensive and requires significant technical expertise.

Here, at MIRO Analytical, we use direct laser absorption spectroscopy (LAS) in the mid-infrared region to monitor O3 together with up to 9 other gases. This technique offers highest sensitivity, selectivity, and real-time monitoring capabilities, making it ideal for trace-level, real-time continuous monitoring. As a robust multi-compound gas analyzer with high time resolution (up to10 Hz), MIRO´s MGA is ideal for airborne measurements in the atmosphere. LAS’ precision, wide dynamic range, non-destructive nature and the direct quantification without conversion needed, contribute to its effectiveness in providing accurate and reliable data. Furthermore, it’s direct measurements of O3 are less susceptible to interference from other gases.
Ozone is a molecule of contrasts—vital in the stratosphere for protecting life on Earth from harmful UV radiation, but dangerous at ground level where it acts as a pollutant. Understanding ozone’s dual role, its sources, and the techniques used to measure it is crucial for managing its impacts on health, the environment, and the climate. Through continued research and monitoring, we can work to mitigate the harmful effects of ground-level ozone while ensuring the recovery of the protective ozone layer. Stay informed and proactive in safeguarding the air we breathe and remember you cannot manage what you cannot measure! Join us next time as we explore another molecule with a story to tell!